Dea 222 Form Example
The dea form 222 is a triplicate form that is required by the dea to allow the exchange of controlled substances from the registrant to another party who is also registered with the dea.
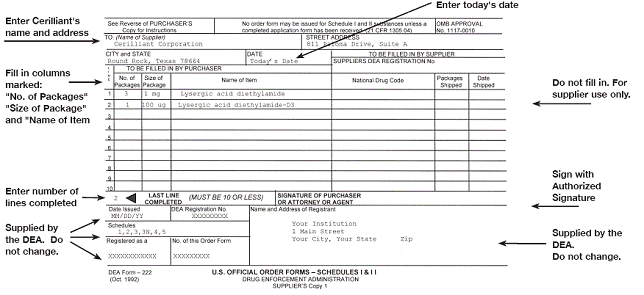
Dea 222 form example. For more information on dea form 222 and to request official order forms. Completed cii order forms dea form 222 for last three years. 1 for a box of 10 x 1 ml syringes 3 for 3 boxes of 25 x 1 ml vials etc. 6 size of package.
10 box 1g 100 btl 1ml 20ml or 50 ml. The drug enforcement administration dea is amending its regulations to implement a new single sheet format for dea form 222 used by dea registrants to order schedules i and ii controlled substances. Partial quantities must be listed on separate lines. 1 of 3 2 of 3 3 of 3.
Community do not mail the completed form to the pharmacy board office. Sample dea form 222. Completed dea form 222 example. Form dea 254 is for individuals requesting to be enrolled for more than one dea registration this addendum form must be associated with a registrant example.
As of october 30 2019 the us drug enforcement administration implemented a new single sheet format for the dea form 222. Purchases then multiply by 100. When the drug enforcement administration dea grants the registrant permission to use schedule i or ii c i or c ii drugs they will send dea form 222 order forms the registrant is responsible for securing the forms 222 and retaining the executed and unexecuted forms. Delaware board of pharmacy.
Delaware board of pharmacy pharmacist in state of delaware. Form dea 254 expires. The rule provides for a two year transition period during which the existing triplicate version of the forms may continue to be used. Quantity per box size of individual vial or container ml g.